37+ Calculating Lattice Energy
This page introduces lattice. The charges of the ions involved and the distance between them.

Born Haber Cycle Basic Introduction Lattice Energy Hess Law Enthalpy Of Formation Chemistry Youtube
Web Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid.

. The total potential energy of. Web How is lattice energy estimated using Born-Haber cycle. Web Lattice Energy -43668-89-05158-4188--328 kJmol -69548 kJmol.
Estimating lattice energy using the Born-Haber cycle has been discussed in Ionic Solids. Web Lattice energy Enthalpy of Formation Electron Affinity Ionization Energy Enthalpy of Sublimation of Positive Ion Enthalpy of Vaporization of Negative ion. Web Lattice energy is influenced by two main factors.
Web Learn the definition of lattice energy see its trends in the Periodic Table and study how to find lattice energy using its formula. Web Both the generation and dissolution of such compounds involve the concept of lattice energy a type of potential energy expressed in units of kJmol. Web Lattice energy is defined as the amount of energy released when cations and anions are brought from infinity to their respective lattice side in a crystal to form one.
U can be calculated from the charges. Choose the cation anion and structure type from the lists provided or choose your own values for the ion charges and radii the. The formula for calculating lattice energy is.
Calculating Lattice Energy of NaCl. Web Some facts The crystal lattice energy is the amount of work energy needed to convert the crystal lattice into ions spaced apart from each other to infinity. The greater the lattice enthalpy the stronger the forces.
Lattice Energy -6418-146-243-737714506-2-349 kJmol -25211 kJmol. Lets consider the example of sodium chloride NaCl to calculate its lattice energy using the Born-Haber cycle. Web I will describe basic types of chemical bonds and then show you how to calculate lattice energy with 3 example problemsCONTENTS000 - Introduction010 -.
Web Lattice energy refers to the energy which is released while two oppositely charged gaseous ions attract to each other and form an ionic solid. Web Physics Formulas Lattice Energy Formula An ionic compound has the overall potential energy which we refer frequently as the lattice energy. Web Lattice energy is a measure of the strength of the ionic bonds in an ionic compound.
Web The lattice energy U of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions. It provides insight into several properties of ionic solids including their volatility their. Web NaCl s Na g Cl g Thus the energy that is required for 1 mole of sodium chloride to separate into gaseous Na and Cl ions is 786 kilojoules.

A E Zfc And Fc Thermomagnetic Curves Of Ni 50 X Mn 37 X Sn Download Scientific Diagram

Chemistry 101 Calculating Lattice Energy Using The Born Haber Cycle Youtube

Chemistry 101 Calculating Lattice Energy Using The Born Haber Cycle Youtube

Born Haber Cycles For Lattice Energy Equation Steps Examples Study Com

How To Use A Born Haber Cycle To Calculate Lattice Enthalpy Youtube

Chemistry Calculating Lattice Energy Youtube

Lattice Energy Al2o3 Youtube

How To Calculate Lattice Enthalpy Using Hess S Law Sum Of Reactions Youtube

Calculate Lattice Energy Le Using The Born Haber Cycle 001 Youtube

Calculated Electron Recombination Rate Black Thick Solid Obtained Download Scientific Diagram
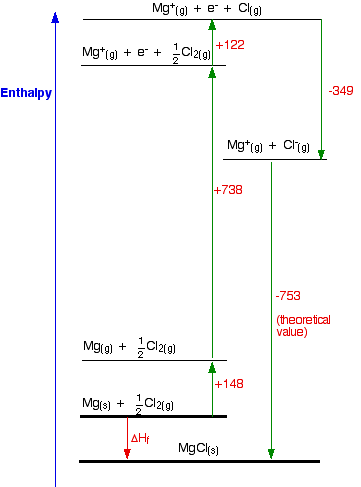
Lattice Enthalpy Lattice Energy

Easiest Way To Calculate Lattice Energy Three Examples Youtube

Chem 101 Calculating Lattice Energy Using The Born Haber Cycle Example 2 Youtube

Chemistry Calculating Lattice Energy Youtube

How To Use A Born Haber Cycle To Calculate Lattice Enthalpy Youtube
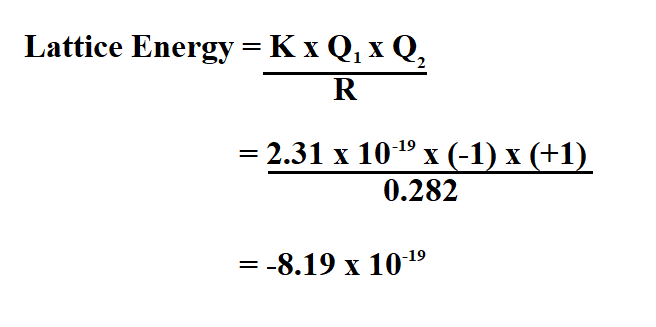
How To Calculate Lattice Energy

Born Haber Cycles For Lattice Energy Equation Steps Examples Study Com